Clinical Study
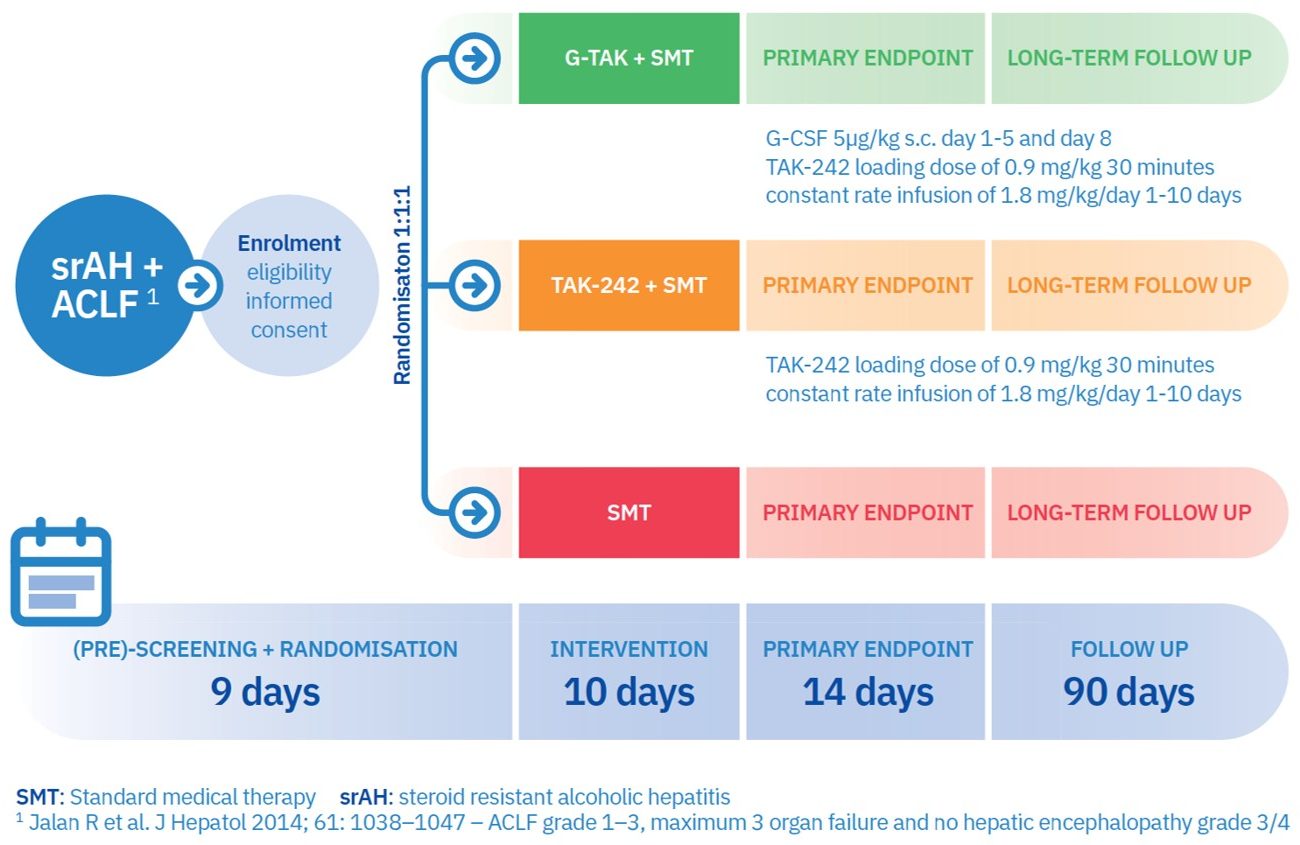
The central objective of A-TANGO is to evaluate G-TAK as a novel therapy of patients with steroid-resistant alcoholic hepatitis (srAH) and acute-on-chronic liver failure (ACLF) in a Phase 2 clinical trial, and to disseminate and exploit the results to bring this novel combinatorial therapy into clinical practice, thereby reducing mortality and increasing the quality of life of cirrhosis patients. Up to 20 liver centres across Europe will participate in this study. Soon, we will add a page where you can view all study sites and search for the closest participating liver centre!
Responsibilities
Charité is the leading clinical study centre providing the principal investigator (PI) of the trial. It is one of Europe’s largest universities and renowned for excellence in medicine and science, having provided half of all German Nobel Prize winners in Physiology or Medicine. The Department for Hepatology and Gastroenterology is one of more than 100 institutes and has specialised on the treatment of all severity grades of liver diseases. It also runs a large liver transplantation programme. EF CLIF coordinates more than 100 clinical centers all over Europe. This network conducts several clinical observational and interventional trials in end-stage liver disease and will be involved in preparing the study protocol and ensuring adequate data management and analysis. UCL was already successful in conducting several liver-health-focused H2020 projects, including ALIVER and CARBALIVE, and will add their expertise in all stages of the trial. It will work with its partner hospital, the Royal Free Hospital to recruit patients. Partner ABR is the contract research organization (CRO) for the clinical trial and is responsible for submission of all required documentation to regulatory authorities, data management, monitoring, and pharmacovigilance, in close collaboration with YAQ, the sponsor of the study. ULEI is a university hospital with extensive experience in conducting interventional trials for liver diseases, including the so-called GRAFT trial which tested granulocyte-colony stimulating factor (G-CSF) in acute-on-chronic liver failure (ACLF). LUMC also has a lot of experience in running clinical trials to treat hepatology-related diseases in patients. All clinical and academic centers that are members of the A-TANGO project (Charité, UCL, ULEI, LUMC) will be involved in preparing and finalizing the study protocol, recruiting patients and conducting the clinical trial at their own liver centres, and supervising the progress of the trial at the other participating study sites in Europe.
Data protection & privacy
Processing of patient data for research within A-TANGO underlies strict ethics guidelines for data protection. Therefore, patient data will only be used after explicit written agreement by each patient and in an untraceable anonymized way.
Contact
For inquiries about the G-TAK trial, the clinical study of the A-TANGO project, please contact the clinical study supervisor Dr. Cornelius Engelmann.