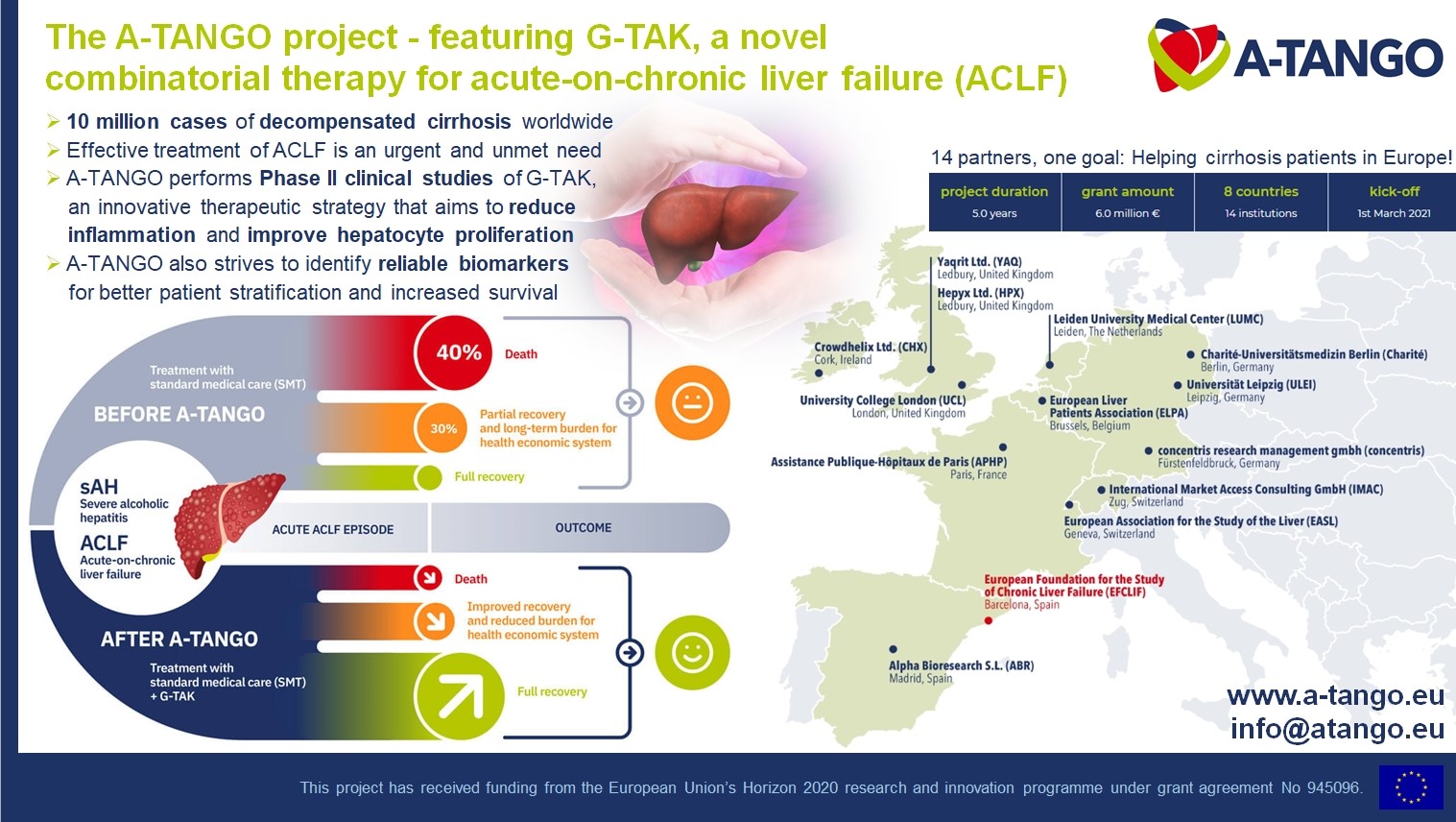
Project Summary
In Europe, about 30,000 people die every year from alcohol-related cirrhosis, a form of chronic, non-communicable disease. The patients that are at highest risk of death are those with superimposed or severe alcoholic hepatitis (sAH) who do not respond to therapy and develop acute-on-chronic liver failure (ACLF), a syndrome characterised by multiorgan failure. Treatment of ACLF is an urgent and unmet need. Based upon existing results of clinical and pre-clinical studies, the A-TANGO consortium will perform Phase 2 clinical trials of a novel, patented and innovative therapeutic strategy by repurposing a toll-like 4 receptor antagonist (TAK242, technology readiness level (TRL) 8), which targets inflammation, in combination with granulocyte colony-stimulating factor (G-CSF, TRL9) that improves hepatocyte proliferation. We call this novel combinatorial therapy “G-TAK” (TRL4). A successful clinical study will advance G-TAK to TRL8. Additionally, A-TANGO aims to discover novel biomarkers for better patient stratification and improved prognosis, to build health economics models, to develop reimbursement strategies, and to disseminate and exploit the therapeutic potential of G-TAK optimally.
The A-TANGO Consortium includes the inventors of G-TAK (UCL, Charité, ULEI and LUMC) and will deliver the project aims through EF CLIF, which has a network of 110 European hospitals. YAQ and HPX are small and medium-sized enterprises (SMEs) that own the background intellectual property (IP) and will ensure study sponsorship and drug supply, while ABR assists with their expertise in clinical trial management, especially with regard to regulatory issues, contracts, and ethics requirements. APHP and IMAC will deliver the economic models. concentris will manage the project, communicate with the project officer at the European Union (EU), and together with EASL, CHX and ELPA engage with patients, initiate widespread dissemination activities, and strive for optimal exploitation of the results. Gender balance will be maintained throughout the project duration.
A-TANGO will achieve the expected impacts of producing meaningful advances in clinical practice by reducing the mortality and improving the quality of life of patients with ACLF whilst reducing disease burden of individual patients and health care systems following validation in these late-stage clinical trials.